Abstract
Introduction: DARA is approved across lines of therapy for multiple myeloma, including in combination with standard-of-care regimens for NDMM. The CXCR4 receptor antagonist plerixafor is used in conjunction with granulocyte colony-stimulating factor (G-CSF) to increase stem cell mobilization for autologous stem cell transplant (ASCT) and can be given by upfront decision or as a rescue strategy. The phase 2 randomized GRIFFIN study (NCT02874742) evaluates frontline DARA in combination with lenalidomide, bortezomib, and dexamethasone (D-RVd) in transplant-eligible NDMM. In the primary analysis, more pts undergoing stem cell mobilization/collection in the D-RVd group received plerixafor compared with the RVd group (69.5% [66/95] vs 56.3% [45/80]) (Voorhees PM, et al. Blood. 2020). The phase 2 MASTER study (NCT03224507) evaluates DARA plus carfilzomib, lenalidomide, and dexamethasone (D-KRd) in transplant-eligible NDMM (Costa LJ, et al. EHA Library. 2020). Here, we present a summary of stem cell mobilization, collection yields, and ASCT data following frontline DARA-based induction therapy in GRIFFIN and MASTER.
Methods: Eligible pts had NDMM and were candidates for ASCT. In GRIFFIN, pts were randomized 1:1 to receive D-RVd or RVd. Pts received 4 induction cycles (21 days) of lenalidomide (R; 25 mg PO on Days 1-14), bortezomib (1.3 mg/m 2 SC on Days 1, 4, 8, and 11), and dexamethasone (d; 40 mg PO QW) ± DARA (16 mg/kg IV QW in Cycles 1-4). After Cycle 4, pts underwent stem cell mobilization with G-CSF ± plerixafor, per institutional standards; if unsuccessful, chemo mobilization was permitted. Pts then received ASCT and subsequently 2 consolidation cycles (21 days) of D-RVd or RVd followed by maintenance therapy with R ± DARA. In the single-arm MASTER study, pts received 4 D-KRd induction cycles, ASCT, and 0, 4 or 8 D-KRd consolidation cycles followed by maintenance therapy with R, based upon achievement of minimal residual disease-negativity. In each 28-day cycle, all pts received carfilzomib (20/56 mg/m 2 IV QW), R (25 mg PO on Days 1-21), d (40 mg PO or IV QW), and DARA (16 mg/kg IV QW for Cycles 1-2, Q2W for Cycles 3-6, and Q4W for Cycles 7+). Mobilization was with G-CSF ± plerixafor as per institutional standards.
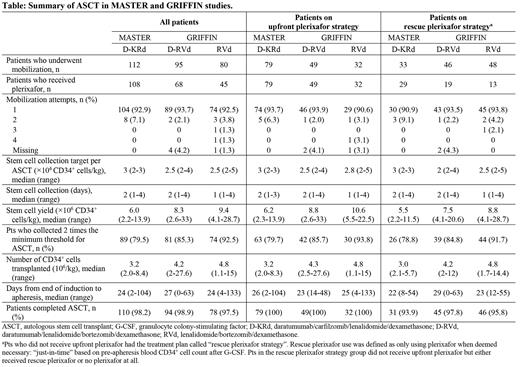
Results: In GRIFFIN, among 207 (D-RVd, n=104; RVd, n=103) randomized pts, 91.3% (n=95) of D-RVd pts and 77.7% (n=80) of RVd pts underwent stem cell mobilization; of those mobilized, 98.9% (n=94) and 97.5% (n=78) underwent ASCT, respectively. In MASTER, 123 D-KRd pts enrolled and at last follow-up, 91.1% (n=112) underwent stem cell mobilization; of those mobilized, 98.2% (n=110) completed ASCT. In GRIFFIN, 46.3% (n=81) of mobilized pts received plerixafor upfront (D-RVd, 51.6%, n=49; RVd, 40.0%, n=32), and 18.3% (n=32 pts) received rescue plerixafor (D-RVd, 20.0%, n=19; RVd, 16.3%, n=13). In MASTER, 70.5% (n=79) D-KRd pts received upfront plerixafor and 25.9% (n=29) received rescue plerixafor. Median CD34 + cell yield was 8.3 × 10 6/kg for D-RVd and 9.4 ×10 6/kg for RVd in GRIFFIN, 6.0 ×10 6/kg for D-KRd in MASTER, and was numerically higher for pts who received upfront plerixafor. Median days for stem cell collection was 1 for pts receiving RVd and 2 for those receiving D-RVd or D-KRd. Median transplanted CD34 + cell count was 4.2 ×10 6/kg for D-RVd and 4.8 ×10 6/kg for RVd in GRIFFIN, and 3.2 ×10 6/kg for D-KRd in MASTER. In GRIFFIN, 93.7% of D-RVd pts and 98.8% of RVd pts reached the minimum institutional CD34 + threshold to perform a single ASCT, which was comparable to results in MASTER (95.5% of D-KRd pts) after first mobilization attempt; 85.3% of D-RVd pts, 92.5% of RVd pts, and 79.5% of D-KRd pts collected 2 times the minimum threshold of stem cells. Additional data by upfront and rescue plerixafor strategies are shown in the Table.
Conclusion: The addition of DARA to proteasome inhibitor/immunomodulatory drug/dexamethasone-based induction therapy has a modest impact on stem cell mobilization, with a lower yield of stem cells and higher median number of days required for collection. Nonetheless, pts were able to undergo transplantation, and most pts collected sufficient stem cells for 2 transplants. Pts who received plerixafor by an upfront decision had numerically higher stem cell yields than pts who received plerixafor by a rescue strategy. An upfront plerixafor strategy for pts receiving DARA-based quadruplet induction therapy should be considered with allowance for additional days of apheresis as needed.
Chhabra: GSK: Honoraria. Costa: Janssen: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau. Kaufman: BMS: Consultancy, Research Funding; Fortis Therapeutics: Research Funding; Roche/Genetech, Tecnopharma: Consultancy, Honoraria; Sutro, Takeda: Research Funding; Genentech, AbbVie, Janssen: Consultancy, Research Funding; Novartis: Research Funding; Incyte, celgene: Consultancy; Tecnofarma SAS, AbbVie: Honoraria; Janssen: Honoraria; Incyte, TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Heidelberg Pharma: Research Funding; Amgen: Research Funding. Sborov: Sanofi: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; SkylineDx: Consultancy; GlaxoSmithKline: Consultancy. Reeves: Incyte Corporation: Honoraria; Takeda: Honoraria; Bristol-Myers Squibb: Speakers Bureau; Pharma Essentia: Consultancy, Honoraria. Rodriguez: Karyopharm: Consultancy, Speakers Bureau; Oncopeptides: Consultancy, Honoraria; Amgen: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. Chari: Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Antengene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shattuck Labs: Consultancy, Membership on an entity's Board of Directors or advisory committees; Secura Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy, Membership on an entity's Board of Directors or advisory committees; Millenium/Takeda: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding. Silbermann: Sanofi Genzyme: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Anderson: Celgene, BMS, Janssen, GSK, Karyopharm, Oncopeptides, Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Shah: Sutro Biopharma: Research Funding; Janssen: Research Funding; Indapta Therapeutics: Consultancy; CareDx: Consultancy; Sanofi: Consultancy; Kite: Consultancy; Poseida: Research Funding; Amgen: Consultancy; BMS/Celgene: Research Funding; Bluebird Bio: Research Funding; CSL Behring: Consultancy; GSK: Consultancy; Precision Biosciences: Research Funding; Teneobio: Research Funding; Oncopeptides: Consultancy; Nektar: Research Funding; Karyopharm: Consultancy. Bumma: Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees. Holstein: Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech, GSK, Janssen, Secura Bio, Sorrento: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Jakubowiak: BMS: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Gracell: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Wildes: Carevive: Consultancy; Seattle Genetics: Consultancy; Sanofi: Consultancy; Janssen: Consultancy. Orlowski: CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Other: Clinical research funding; Asylia Therapeutics, Inc., BioTheryX, Inc., and Heidelberg Pharma, AG.: Other: Laboratory research funding; Asylia Therapeutics, Inc.: Current holder of individual stocks in a privately-held company, Patents & Royalties; Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, Forma Therapeutics, Genzyme, GSK Biologicals, Janssen Biotech, Juno Therapeutics, Karyopharm Therapeutics, Inc., Kite Pharma, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, I: Membership on an entity's Board of Directors or advisory committees; Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, EcoR1 Capital LLC, Genzyme, GSK Biologicals, Janssen Biotech, Karyopharm Therapeutics, Inc., Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda P: Consultancy, Honoraria. Shain: Novartis Pharmaceuticals Corporation: Consultancy; Karyopharm Therapeutics Inc.: Honoraria, Research Funding; Janssen oncology: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi Genzyme: Consultancy, Speakers Bureau; GlaxoSmithLine, LLC: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Adaptive Biotechnologies Corporation: Consultancy, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cowan: Janssen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; Cellectar: Consultancy; Harpoon: Research Funding; GSK: Consultancy; Secura Bio: Consultancy; BMS: Research Funding; Nektar: Research Funding. Dholaria: Takeda: Research Funding; Jazz: Speakers Bureau; MEI: Research Funding; Angiocrine: Research Funding; Poseida: Research Funding; Celgene: Speakers Bureau; Pfizer: Research Funding; Janssen: Research Funding. Pei: Janssen: Current Employment, Current equity holder in publicly-traded company. Cortoos: Janssen: Current Employment, Current equity holder in publicly-traded company. Patel: Janssen: Current Employment. Bartlett: Janssen: Current Employment. Vermeulen: Janssen: Current Employment, Current equity holder in publicly-traded company. Lin: Janssen: Current Employment. Richardson: AstraZeneca: Consultancy; Regeneron: Consultancy; Celgene/BMS: Consultancy, Research Funding; Oncopeptides: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; AbbVie: Consultancy; GlaxoSmithKline: Consultancy; Karyopharm: Consultancy, Research Funding; Protocol Intelligence: Consultancy; Janssen: Consultancy; Sanofi: Consultancy; Secura Bio: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding. Voorhees: Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Secura Bio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
The specific regimen combination is not yet approved, but individual components are.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal